Volume 20, Issue 1 (Spring 2025)
Salmand: Iranian Journal of Ageing 2025, 20(1): 2-19 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Assadiasl S, Safdel S, Hossein Nicknam M. A Review of Senolytic Drugs in Reducing the Biological Problems of Aging. Salmand: Iranian Journal of Ageing 2025; 20 (1) :2-19
URL: http://salmandj.uswr.ac.ir/article-1-2767-en.html
URL: http://salmandj.uswr.ac.ir/article-1-2767-en.html
1- Molecular Immunology Research Center, Tehran University of Medical Sciences, Tehran, Iran. & Iranian Tissue Bank and Research Center, Tehran University of Medical Sciences, Tehran, Iran. , assadiasl@sina.tums.ac.ir
2- Molecular Immunology Research Center, Tehran University of Medical Sciences, Tehran, Iran.
3- Molecular Immunology Research Center, Tehran University of Medical Sciences, Tehran, Iran. & Department of Immunology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
2- Molecular Immunology Research Center, Tehran University of Medical Sciences, Tehran, Iran.
3- Molecular Immunology Research Center, Tehran University of Medical Sciences, Tehran, Iran. & Department of Immunology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 6897 kb]
(2125 Downloads)
| Abstract (HTML) (5250 Views)
Full-Text: (1065 Views)
Introduction
Considering the increase in the elderly population and aging-related disorders, there is a need for strategies to reduce the complications of aging. Senolytic agents are a group of different drugs that improve the function of tissues and organs in elderly people by selective elimination of aging cells, mainly by inducing apoptosis in cells with senescent phenotype [2]. These drugs have been developed for therapeutic purposes other than senolysis; however, in many laboratory, experimental, and clinical studies, senolytic properties have been observed in them. In this review article, a summary of the mechanisms of cellular aging and the selective effect of senolytic agents on these cells, as well as different categories of senolytic drugs and therapeutic strategies with senolytic effect, the results of their administration, and the progress made in this field have been provided.
Methods & Materials
According to the publication of the first articles about senolytics in 2015, all of the articles with the keywords “senolysis” or “senolytics” in title or abstract published between 2015 and 2023 were searched in PubMed, Scopus, and Google Scholar search engines. Subsequently, 108 out of 121 articles, which were in English and open access, were downloaded and assessed. After studying the articles and validating them, repetitive articles, review articles about a specific disorder, editorials, case reports, and articles published in non-indexed journals and congress booklets were omitted (considering the PRISMA Checklist of systematic review articles). As a result, 50 articles remained, and a summary of their findings was provided as a review article.
Results
Currently, 12 categories of senolytic agents have been identified, and their therapeutic effects in amelioration of aging complications are under study [6]. The first category is kinase inhibitors, from which dasatinib has shown the most anti-senescence effects. The second category is natural polyphenols, including quercetin, which is the main flavonoid found in vegetables and fruits. The combination of dasatinib and quercetin has shown the most efficacy in reducing the effects of aging. Various anti-cancer drugs and those that induce apoptosis in cells, including Bcl2 protein family inhibitors and P53 stabilizers, are the other categories of senolytic drugs. Navitoclax and UBX0101 are the most studied drugs in these two categories, respectively, which have caused a decrease in the number of senescent cells in joint and liver tissues of the experimental models.
The next groups include heat shock protein inhibitors and inhibitors of the BET protein family, which have been investigated for their anti-inflammatory and anti-cancer effects in vitro as well as in preclinical studies. Administration of these drugs resulted in the apoptosis of cells with aging phenotype in human and experimental models.
Cardiac steroids are mainly used in the treatment of heart failure; however, digoxin and ouabain were effective in the selective elimination of aged fibroblasts and apoptosis of senescent liver cells. PPARα agonists, such as fenofibrate, have also shown therapeutic effects in osteoarthritis and cartilage degeneration. In addition, antibiotics, such as azithromycin and roxithromycin, have been able to eliminate senescent fibroblasts in laboratory studies, but studies on the senolytic effects of other antibiotics are ongoing. Moreover, integrase inhibitors classified as antiviral drugs induced apoptosis in old T lymphocytes and may be used as senolytic agents [7].
The other strategy that has been proposed to remove old cells is calorie restriction. Fasting and calorie restriction have already shown positive effects in rejuvenating tissues and increasing life span. This alternative has significantly induced younger genetic profiles in human and animal studies [48]. Furthermore, novel therapeutic approaches, such as chimeric antigen receptor (CAR-T) cell therapy, have the potential to selectively eliminate aged cells that express senescence markers. These strategies are being studied in various phases of preclinical or clinical research (Table 1) [9].
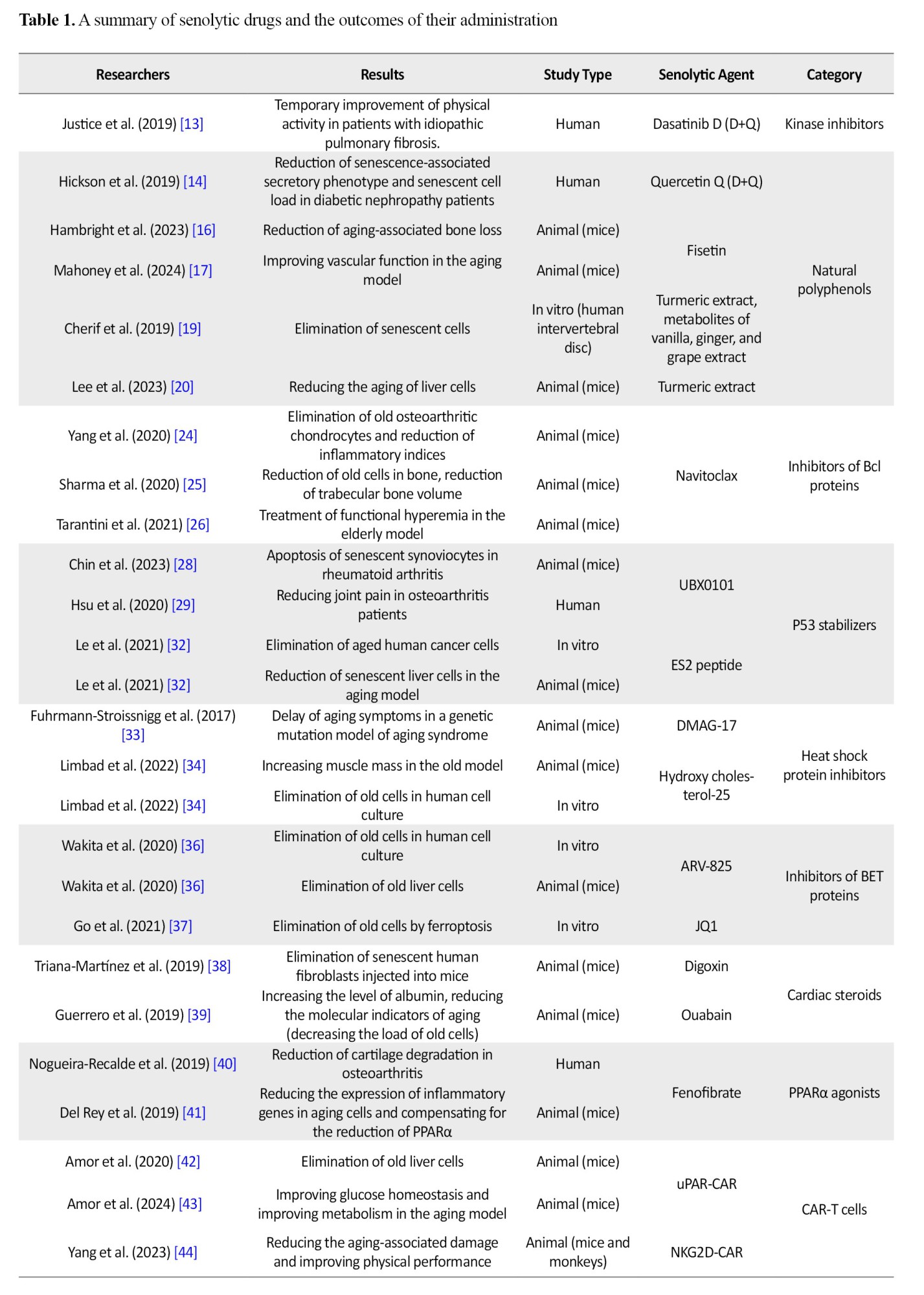
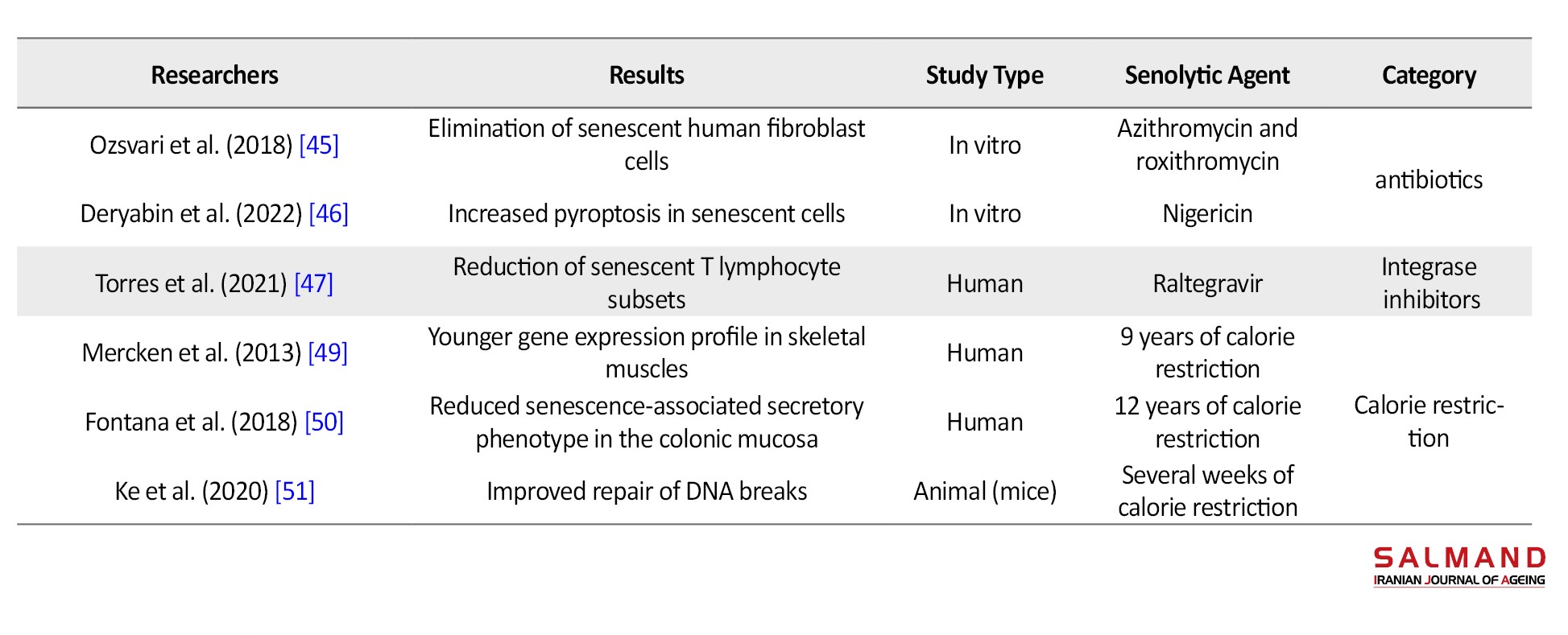
Conclusion
Considering the increasing trend in the elderly population, one of the solutions to deal with debilitating complications of old age is senolytic drugs, which can eliminate senescent cells with unfavorable function. The main categories of senolytics with the most promising results are kinase inhibitors and natural polyphenols; however, new strategies, such as specific targeting of senescent cells using CAR-T cells technology, are also under study. Although in various research models, senolytics have shown efficacy in reducing the complications of aging, a standard treatment regimen has not yet been established, and studies are still ongoing. If the effectiveness and safety of these drugs in removing senescent cells are confirmed, there will be the possibility of providing a healthier and more dynamic aging for the elderly population in the future.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Drafting the manuscript: Sara Assadiasl; Search and data collection: Sepehr Safdel; Revision and edition of the manuscript: Mohammad Hossein Nicknam
Conflicts of interest
The authors declared no conflict of interest.
References
Considering the increase in the elderly population and aging-related disorders, there is a need for strategies to reduce the complications of aging. Senolytic agents are a group of different drugs that improve the function of tissues and organs in elderly people by selective elimination of aging cells, mainly by inducing apoptosis in cells with senescent phenotype [2]. These drugs have been developed for therapeutic purposes other than senolysis; however, in many laboratory, experimental, and clinical studies, senolytic properties have been observed in them. In this review article, a summary of the mechanisms of cellular aging and the selective effect of senolytic agents on these cells, as well as different categories of senolytic drugs and therapeutic strategies with senolytic effect, the results of their administration, and the progress made in this field have been provided.
Methods & Materials
According to the publication of the first articles about senolytics in 2015, all of the articles with the keywords “senolysis” or “senolytics” in title or abstract published between 2015 and 2023 were searched in PubMed, Scopus, and Google Scholar search engines. Subsequently, 108 out of 121 articles, which were in English and open access, were downloaded and assessed. After studying the articles and validating them, repetitive articles, review articles about a specific disorder, editorials, case reports, and articles published in non-indexed journals and congress booklets were omitted (considering the PRISMA Checklist of systematic review articles). As a result, 50 articles remained, and a summary of their findings was provided as a review article.
Results
Currently, 12 categories of senolytic agents have been identified, and their therapeutic effects in amelioration of aging complications are under study [6]. The first category is kinase inhibitors, from which dasatinib has shown the most anti-senescence effects. The second category is natural polyphenols, including quercetin, which is the main flavonoid found in vegetables and fruits. The combination of dasatinib and quercetin has shown the most efficacy in reducing the effects of aging. Various anti-cancer drugs and those that induce apoptosis in cells, including Bcl2 protein family inhibitors and P53 stabilizers, are the other categories of senolytic drugs. Navitoclax and UBX0101 are the most studied drugs in these two categories, respectively, which have caused a decrease in the number of senescent cells in joint and liver tissues of the experimental models.
The next groups include heat shock protein inhibitors and inhibitors of the BET protein family, which have been investigated for their anti-inflammatory and anti-cancer effects in vitro as well as in preclinical studies. Administration of these drugs resulted in the apoptosis of cells with aging phenotype in human and experimental models.
Cardiac steroids are mainly used in the treatment of heart failure; however, digoxin and ouabain were effective in the selective elimination of aged fibroblasts and apoptosis of senescent liver cells. PPARα agonists, such as fenofibrate, have also shown therapeutic effects in osteoarthritis and cartilage degeneration. In addition, antibiotics, such as azithromycin and roxithromycin, have been able to eliminate senescent fibroblasts in laboratory studies, but studies on the senolytic effects of other antibiotics are ongoing. Moreover, integrase inhibitors classified as antiviral drugs induced apoptosis in old T lymphocytes and may be used as senolytic agents [7].
The other strategy that has been proposed to remove old cells is calorie restriction. Fasting and calorie restriction have already shown positive effects in rejuvenating tissues and increasing life span. This alternative has significantly induced younger genetic profiles in human and animal studies [48]. Furthermore, novel therapeutic approaches, such as chimeric antigen receptor (CAR-T) cell therapy, have the potential to selectively eliminate aged cells that express senescence markers. These strategies are being studied in various phases of preclinical or clinical research (Table 1) [9].


Conclusion
Considering the increasing trend in the elderly population, one of the solutions to deal with debilitating complications of old age is senolytic drugs, which can eliminate senescent cells with unfavorable function. The main categories of senolytics with the most promising results are kinase inhibitors and natural polyphenols; however, new strategies, such as specific targeting of senescent cells using CAR-T cells technology, are also under study. Although in various research models, senolytics have shown efficacy in reducing the complications of aging, a standard treatment regimen has not yet been established, and studies are still ongoing. If the effectiveness and safety of these drugs in removing senescent cells are confirmed, there will be the possibility of providing a healthier and more dynamic aging for the elderly population in the future.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Drafting the manuscript: Sara Assadiasl; Search and data collection: Sepehr Safdel; Revision and edition of the manuscript: Mohammad Hossein Nicknam
Conflicts of interest
The authors declared no conflict of interest.
References
- World Health Organization. WHO clinical consortium on healthy ageing 2022: Report of consortium meeting, 5-6 December 2022. Geneva: World Health Organization; 2023. [Link]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015; 14(4):644-58. [DOI:10.1111/acel.12344] [PMID]
- Gonzales MM, Garbarino VR, Kautz TF, Palavicini JP, Lopez-Cruzan M, Dehkordi SK, et al. Senolytic therapy in mild Alzheimer’s disease: A phase 1 feasibility trial. Nature Medicine. 2023; 29(10):2481-8. [DOI:10.1038/s41591-023-02543-w] [PMID]
- Riessland M, Orr ME. Translating the biology of aging into new therapeutics for Alzheimer’s Disease: Senolytics. The Journal of Prevention of Alzheimer’s Disease. 2023; 10(4):633-46. [DOI:10.14283/jpad.2023.104] [PMID]
- Muñoz-Espín D, Serrano M. Cellular senescence: From physiology to pathology. Nature Reviews Molecular Cell Biology. 2014; 15(7):482-96. [DOI:10.1038/nrm3823] [PMID]
- Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: The path to the clinic. Nature Medicine. 2022; 28(8):1556-68. [DOI:10.1038/s41591-022-01923-y] [PMID]
- Kirkland JL, Tchkonia T. Cellular senescence: A translational perspective. EBioMedicine. 2017; 21:21-8. [DOI:10.1016/j.ebiom.2017.04.013] [PMID]
- Kang C. Senolytics and senostatics: A two-pronged approach to target cellular senescence for delaying aging and age-related diseases. Molecules and Cells. 2019; 42(12):821-7. [PMID]
- Carney EF. Use of CAR T cells as senolytic agents. Nature Reviews Nephrology. 2020; 16(9):485. [DOI:10.1038/s41581-020-0324-3] [PMID]
- Assadiasl S, Fatahi Y, Mosharmovahed B, Mohebbi B, Nicknam MH. Baricitinib: From Rheumatoid Arthritis to COVID‐19. The Journal of Clinical Pharmacology. 2021; 61(10):1274-85. [DOI:10.1002/jcph.1874] [PMID]
- Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. Journal of Pharmacology and Experimental Therapeutics. 2005; 315(3):971-9. [DOI:10.1124/jpet.105.084145] [PMID]
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. New England Journal of Medicine. 2006; 354(24):2531-41. [DOI:10.1056/NEJMoa055229] [PMID]
- Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019; 40:554-63. [DOI:10.1016/j.ebiom.2018.12.052] [PMID]
- Hickson LJ, Prata LGL, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019; 47:446-56. [DOI:10.1016/j.ebiom.2019.08.069] [PMID]
- Khan N, Syed DN, Ahmad N, Mukhtar H. Fisetin: A dietary antioxidant for health promotion. Antioxidants & Redox Signaling. 2013; 19(2):151-62. [DOI:10.1089/ars.2012.4901] [PMID]
- Hambright WS, Mu X, Gao X, Guo P, Kawakami Y, Mitchell J, et al. The senolytic drug fisetin attenuates bone degeneration in the zmpste24-/- progeria mouse model. Journal of Osteoporosis. 2023; 2023:5572754. [DOI:10.1155/2023/5572754] [PMID]
- Mahoney SA, Venkatasubramanian R, Darrah MA, Ludwig KR, VanDongen NS, Greenberg NT, et al. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence. Aging Cell. 2024; 23(3):e14060. [DOI:10.1111/acel.14060] [PMID]
- Verdoorn BP, Evans TK, Hanson GJ, Zhu Y, Langhi Prata LGP, Pignolo RJ, et al. Fisetin for COVID‐19 in skilled nursing facilities: Senolytic trials in the COVID era. Journal of the American Geriatrics Society. 2021; 69(11):3023-33. [DOI:10.1111/jgs.17416] [PMID]
- Cherif H, Bisson DG, Jarzem P, Weber M, Ouellet JA, Haglund L. Curcumin and o-Vanillin exhibit evidence of senolytic activity in human IVD cells in vitro. Journal of Clinical Medicine. 2019; 8(4):433. [DOI:10.3390/jcm8040433] [PMID]
- Lee DY, Lee SJ, Chandrasekaran P, Lamichhane G, O'Connell JF, Egan JM, et al. Dietary Curcumin Attenuates Hepatic Cellular Senescence by Suppressing the MAPK/NF-κB Signaling Pathway in Aged Mice. Antioxidants. 2023; 12(6):1165. [DOI:10.3390/antiox12061165] [PMID]
- Troiani M, Colucci M, D’Ambrosio M, Guccini I, Pasquini E, Varesi A, et al. Single-cell transcriptomics identifies Mcl-1 as a target for senolytic therapy in cancer. Nature Communications. 2022; 13(1):2177. [DOI:10.1038/s41467-022-29824-1] [PMID]
- Mohamad Anuar NN, Nor Hisam NS, Liew SL, Ugusman A. Clinical review: Navitoclax as a pro-apoptotic and anti-fibrotic agent. Frontiers in Pharmacology. 2020; 11:564108. [DOI:10.3389/fphar.2020.564108] [PMID]
- Jia K, Dai Y, Liu A, Li X, Wu L, Lu L, et al. Senolytic agent navitoclax inhibits angiotensin II-induced heart failure in mice. Journal of Cardiovascular Pharmacology. 2020; 76(4):452-60. [DOI:10.1097/FJC.0000000000000878] [PMID]
- Yang H, Chen C, Chen H, Duan X, Li J, Zhou Y, et al. Navitoclax (ABT263) reduces inflammation and promotes chondrogenic phenotype by clearing senescent osteoarthritic chondrocytes in osteoarthritis. Aging (Albany NY). 2020; 12(13):12750. [DOI:10.18632/aging.103177] [PMID]
- Sharma AK, Roberts RL, Benson RD Jr, Pierce JL, Yu K, Hamrick MW, et al. The senolytic drug navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice. Frontiers in Cell and Developmental Biology. 2020; 8:354. [DOI:10.3389/fcell.2020.00354] [PMID]
- Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. GeroScience. 2021; 43(5):2427-40. [DOI:10.1007/s11357-021-00440-z] [PMID]
- González-Gualda E, Pàez-Ribes M, Lozano-Torres B, Macias D, Wilson JR 3rd, González-López C, et al. Galacto‐conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell. 2020; 19(4):e13142. [DOI:10.1111/acel.13142] [PMID]
- Chin AF, Han J, Clement CC, Choi Y, Zhang H, Browne M, et al. Senolytic treatment reduces oxidative protein stress in an aging male murine model of post‐traumatic osteoarthritis. Aging Cell. 2023; 22(11):e13979. [DOI:10.1111/acel.13979] [PMID]
- Hsu B, Visich J, Lane N, Li L, Mittal J, An M, et al. Safety, tolerability, pharmacokinetics, and clinical outcomes following treatment of painful knee osteoarthritis with senolytic molecule UBX0101. Osteoarthritis and Cartilage. 2020; 28:S479-S80. [DOI:10.1016/j.joca.2020.02.752]
- Lane N, Hsu B, Visich J, Xie B, Khan A, Dananberg J. A phase 2, randomized, double-blind, placebo-controlled study of senolytic molecule UBX0101 in the treatment of painful knee osteoarthritis. Osteoarthritis and Cartilage. 2021; 29(1):S52-3. [DOI:10.1016/j.joca.2021.02.077]
- Wiley CD, Schaum N, Alimirah F, Lopez-Dominguez JA, Orjalo AV, Scott G, et al. Small-molecule MDM2 antagonists attenuate the senescence-associated secretory phenotype. Scientific Reports. 2018; 8(1):2410. [DOI:10.1038/s41598-018-20000-4] [PMID]
- Le HH, Cinaroglu SS, Manalo EC, Ors A, Gomes MM, Sahbaz BD, et al. Molecular modelling of the FOXO4-TP53 interaction to design senolytic peptides for the elimination of senescent cancer cells. EBioMedicine. 2021; 73:103646. [DOI:10.1016/j.ebiom.2021.103646] [PMID]
- Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nature Communications. 2017; 8(1):422. [DOI:10.1038/s41467-017-00314-z] [PMID]
- Limbad C, Doi R, McGirr J, Ciotlos S, Perez K, Clayton ZS, et al. Senolysis induced by 25-hydroxycholesterol targets CRYAB in multiple cell types. iScience. 2022; 25(2):103848. [DOI:10.1016/j.isci.2022.103848] [PMID]
- Cheung KL, Kim C, Zhou MM. The functions of BET Proteins In Gene Transcription Of Biology And Diseases. Frontiers in Molecular Biosciences. 2021; 8:728777. [DOI:10.3389/fmolb.2021.728777] [PMID]
- Wakita M, Takahashi A, Sano O, Loo TM, Imai Y, Narukawa M, et al. A BET family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Nature Communications. 2020; 11(1):1935. [DOI:10.1038/s41467-020-15719-6] [PMID]
- Go S, Kang M, Kwon SP, Jung M, Jeon OH, Kim BS. The senolytic drug JQ1 removes senescent cells via ferroptosis. Tissue Engineering and Regenerative Medicine. 2021; 18(5):841-50. [DOI:10.1007/s13770-021-00346-z] [PMID]
- Triana-Martínez F, Picallos-Rabina P, Da Silva-Álvarez S, Pietrocola F, Llanos S, Rodilla V, et al. Identification and characterization of Cardiac Glycosides as senolytic compounds. Nature Communications. 2019; 10(1):4731. [DOI:10.1038/s41467-019-12888-x] [PMID]
- Guerrero A, Herranz N, Sun B, Wagner V, Gallage S, Guiho R, et al. Cardiac glycosides are broad-spectrum senolytics. Nature Metabolism. 2019; 1(11):1074-88. [DOI:10.1038/s42255-019-0122-z] [PMID]
- Nogueira-Recalde U, Lorenzo-Gómez I, Blanco FJ, Loza MI, Grassi D, Shirinsky V, et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine. 2019; 45:588-605. [DOI:10.1016/j.ebiom.2019.06.049] [PMID]
- Del Rey MJ, Valín Á, Usategui A, Ergueta S, Martín E, Municio C, et al. Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immunity & Ageing : I & A. 2019; 16:29. [DOI:10.1186/s12979-019-0169-4] [PMID]
- Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020; 583(7814):127-32. [DOI:10.1038/s41586-020-2403-9] [PMID]
- Amor C, Fernández-Maestre I, Chowdhury S, Ho YJ, Nadella S, Graham C, et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nature Aging. 2024; 4:336–49. [Link]
- Yang D, Sun B, Li S, Wei W, Liu X, Cui X, et al. NKG2D-CAR T cells eliminate senescent cells in aged mice and nonhuman primates. Science Translational Medicine. 2023; 15(709):eadd1951. [DOI:10.1126/scitranslmed.add1951] [PMID]
- Ozsvari B, Nuttall JR, Sotgia F, Lisanti MP. Azithromycin and Roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging (Albany NY). 2018; 10(11):3294-307. [DOI:10.18632/aging.101633] [PMID]
- Deryabin PI, Shatrova AN, Borodkina AV. Targeting multiple homeostasis-maintaining systems by ionophore Nigericin Is a novel approach for senolysis. International Journal of Molecular Sciences. 2022; 23(22):14251. [DOI:10.3390/ijms232214251] [PMID]
- Torres B, Guardo A, Squarcia M, Diaz A, Fabra A, Caballero M, et al. Impact of switching to raltegravir and/or adding losartan in lymphoid tissue fibrosis and inflammation in people living with HIV. A randomized clinical trial. HIV Medicine. 2021; 22(8):674-81. [DOI:10.1111/hiv.13114] [PMID]
- Fontana L, Nehme J, Demaria M. Caloric restriction and cellular senescence. Mechanisms of Ageing and Development. 2018; 176:19-23. [DOI:10.1016/j.mad.2018.10.005] [PMID]
- Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013; 12(4):645-51. [DOI:10.1111/acel.12088] [PMID]
- Fontana L, Mitchell SE, Wang B, Tosti V, van Vliet T, Veronese N, et al. The effects of graded caloric restriction: XII. Comparison of mouse to human impact on cellular senescence in the colon. Aging Cell. 2018; 17(3):e12746. [DOI:10.1111/acel.12746] [PMID]
- Ke Z, Firsanov D, Spencer B, Seluanov A, Gorbunova V. Short-term calorie restriction enhances DNA repair by non-homologous end joining in mice. NPJ Aging and Mechanisms of Disease. 2020; 6:9. [DOI:10.1038/s41514-020-00047-2] [PMID]
Type of Study: Research |
Subject:
Geriatric
Received: 2024/01/06 | Accepted: 2024/03/03 | Published: 2025/04/01
Received: 2024/01/06 | Accepted: 2024/03/03 | Published: 2025/04/01
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








